Sterilizing Filters Have A Pore Size Of 0.22 Þâ¼m. Which Of The Following Statements Is True?
1. Introduction
The incidence of cancer remains mutual in spite of the discoveries in the field of tumor cells, molecular biology, and a variety of surgical, radiation, and pharmacological intervention methods on various stages and components of tumor growth, which is why it is important to create effective anticancer drugs and improve existing therapies [1]. Therefore, there is now an ongoing search for original drug substances (DS), which is based on conveying out chemic, physical, biological, and pharmaceutical studies [2].
One of the priority directions of inquiry in this area is the development of new anticancer drugs derived from nitrosoureas (NUs) that vest to alkylating agents. A feature characteristic of these compounds that contributes to their biological activity is a hydrolytic decomposition in torso conditions with a germination of alkylating and carbamoylating particles, and a lack of cantankerous-resistance to typical alkylating agents. The search for new active compounds of NU is associated with attempts to expand the range of the antitumor event, to reduce side furnishings and toxicity, and to increase the selectivity of activity [3,4,5,6].
The Institute of Organic Synthesis has locally synthesized a new substance fromthe NU class—ormustine. In preliminary biological experiments carried out in the Federal Country Budgetary Scientific Institution, the N.N. Blokhin Russian Cancer Research Center, it was found that ormustine induces the death of tumor cells past the mechanism of early on apoptosis; moreover, on animals, information technology exhibits high dose-dependent antitumor activity [7,8,9,10].
Thus, the cosmos of a stable and effective pharmaceutical composition (PF) of ormustine is a highly relevant area of research.
2. Results and Discussion
2.i. The Choice of Solvent for the Substance of Ormustine
Since the most constructive anticancer drugs have an injectable class of PF, ormustine (Figure one) was besides developed for intravenous injection initially as a solution. Since this substance is slowly and sparingly soluble in h2o, to increase its solubility, different solvents permitted for use in injectable PF were used: 5% solution of mannitol, 2% solutions of Kollidon 17 PF, Kollidon 12 PF and dextran (Mr~70,000), 10% solution of PEG-1500 and Kollisolv PEG-400, 0.2%, 2%, iv%, and six% solutions of citric acrid; 0.1 Thou hydrochloric acid (Table ane).
Tabular array one shows that a significant increase in the solubility by two times or more was achieved only with the utilise of citric and hydrochloric acids. However, preliminary studies with the received biological solutions institute that the introduction of the citric acid solution at a concentration of 2%–6% to laboratory animals causes decease associated with hemolytic reaction [8]. Therefore, nosotros chose to use a 0.ane M hydrochloric acid solution to increment the solubility of the ormustine substance.
2.2. The Choice of Method of Ormustine Dissolution in 0.1 M Hydrochloric acid
Later on the concluding choice of a solvent for ormustine substance, we studied the effect of different solubility intensification techniques—heat to 50 °C, ultrasonic treatment, stirring with magnetic or propeller stirrer—to reduce the time of dissolution and avoid a significant reduction in the concentration of the active substance in solution (Tabular array two).
To make up one's mind the dissolution rate of ormustine in 0.1 M hydrochloric acid, 100 mL of solvent was taken and 2.v one thousand of the substance was gradually dissolved therein, determining the amount of solute every ten min until complete dissolution, afterwards which the content of the agile substance was evaluated in the solution.
Information presented in Tabular array 2 point that the use of ultrasound in the PF preparation procedure can significantly accelerate the dissolving of the substance and thus maintains the concentration of the active substance practically at baseline. A quicker dissolution of an active ingredient under the influence of the ultrasound is bound to the fact that, nether the influence of ultrasonic microstreams, in that location is an intensive interfusion of liquid layers at the surface. The rate of dissolution of ormustine nether the influence of the ultrasound is besides increased every bit a result of cavitational erosion and the adding of solid particles. This considerably increases the interface between the dissolvent and the dissolved substance. While intensification of the substance dissolving using a magnetic propeller stirrer and heating but slightly accelerates the dissolution process, it reduces the concentration of the active substance by more than 10% of the original. Therefore, we can conclude the feasibility of the use of ultrasound technology for ormustine PF.
ii.3. Choice of Shaper for Lyophilization of Ormustine Solution
A ii.five% solution of ormustine is unstable during storage; within 24 h, there is a subtract in the concentration of the active substance by 3%–6%; therefore, to stabilize and increment the shelf life of PF, it is necessary to conduct lyophilization. Therefore, we examined the effect ofthe inclusion of a shaper in the ormustine solution during freeze drying to form a porous lyophilic mass and provide an absence of pregnant reduction in the active substance content in the solution during process operations (at least 3 h). During the production of the model compounds, the ormustine substance was dissolved in 0.1 M hydrochloric acid under ultrasound activity. After complete dissolution of the substance, 1 or more auxiliary substances were added to DS: Kollidon 12 PF and 17 PF, lactose, mannitol, and citric acid at diverse concentrations (Table 3). Then, it was filtered through a nylon filter membrane.
The results presented in Table 3 indicate that the improver of 6% Kollidon 17 PF every bit a shaper and cosolvent in the model solution compared with the ii.5% ormustine solution without auxiliaries significantly reduced the loss of DS from seven.three% to 1.4% during the grooming of the solution for lyophilization. The solution containing a mixture of half-dozen% Kollidon 12 PF and 0.ane% citric acrid showed a slight decrease of ormustine losses, while but the addition of 10% Kollidon 12 PF or a mixture of iv% Kollidon 12 PF and 2% lactose destabilized, on the contrary, the active substance by one.v times. The improver of 4% lactose and 4% mannitol led to an increased degradation of DS by about 2.v times. In connection to the above, Kollidon 17 PF at a concentration of 6% was chosen as the most optimal ormustine shaper stabilizing solution.
2.4. Development of Lyophilization Mode for the Solution of Ormustine
To stabilize ormustine the PF solution, some trial lyophilizations were conducted under various atmospheric condition. We evaluated the effectiveness of the proposed mode on the parameters: lyophilization duration and the quality of the obtained lyophilizate (appearance, pH change, loss of DS during lyophilization, and humidity). Beingness a government allowing obtainment of the lyophilizate with a adept advent, with lilliputian loss of the active ingredient in the shortest time, we selected a fashion in which the conception was frozen at −45 °C on a shelf of a freeze drying unit of measurement (a shelf temperature of −45 °C) and maintained this temperature for 3 h; then, the vacuum was switched on. After stabilization of the vacuum, the condenser, and the conception temperature, heating was performed at the charge per unit of +5 °C/h up to a shelf temperature of −35 °C. Further temperature increase (afterward passage of the conception eutectic zone that is −23 °C) was at +3 °C per hour. Upon reaching 0 °C on the formulation, we performed a gradual ascension in temperature to +twenty °C at +2 °C/h; after reaching a predetermined temperature for the preparation, a final drying (removal of residual wet) was carried out over the course of iii h (Figure 2).
two.5. The Pick of Solvent for Rehydrating of Lyophilized Ormustine PF Prior to Assistants
At the final phase of the technological enquiry, information technology was necessary to cull a solvent for the rehydrating of the freeze dried production. For this purpose, nosotros evaluated the effect of solutions virtually commonly used for injecting (0.9% NaCl solution, 5% glucose solution, Ringer's solution, Hemodes-North, and phosphate buffered saline with a pH of half-dozen.8–seven.ane) on pH and the concrete stability of the solution obtained later rehydration of the ormustine lyophilizate vial (Tabular array four).
All solvents provided a true solution, only a significant increase in pH was ensured only through the awarding of a phosphate buffer solution. Further, in social club to select the nigh appropriate solvent from the five proposed options, we assessed their touch on on the stability of the resulting ormustine in the solution subsequently rehydration. For this purpose, ormustine lyophilizate was dissolved in v mL of a 0.ix% NaCl solution, a 5% glucose solution, a Ringer's solution, Hemodes-N, and 10 mL of a phosphate buffer solution, and the concentration of active compound was measured at control fourth dimension intervals—ane h, three h, eight h, and 24 h. (Figure 3).
When Ringer'due south and Hemodez-Due north solutions were used as solvents, 1 h later rehydration of lyophilizate, we observed a reduction in the concentration of the agile substance; losses were iv% and eight%, respectively. After 1 twenty-four hours, the ormustine concentration in the solution with the to a higher place-mentioned solvents fell to twoscore% of the original. The use of the isotonic sodium chloride solution and the phosphate buffer solution equally solvents allowed us to keep the original concentration of ormustine for 1 h and of the v% glucose solution for 3 h. The everyman losses of the agile substance at all stages of the experiment were observed with the employ of the 5% glucose solution and the phosphate buffer solution as solvents. Therefore, further studies for the rehydrating of freeze dried product could use a 5% glucose solution, a phosphate buffer solution, and a 0.9% solution of NaCl.
2.6. Results of Preliminary Preclinical Trials
From the results of several preliminary preclinical trials, information technology was established that, past unmarried intravenous medication assistants, ormustine in a 125 mg/kg dose led to the successful handling of mice with leukoses in a large percent of cases. In regard to P-388 lymphocytic leukemia, ormustine led to the handling of mice in l% of cases; in regard to 50-1210 lymphoid leukemia, information technology was 66.vii%; in regard to the cervical cancer (CC), ormustine led to the treatment of all mice in the experimental grouping. At the same fourth dimension, extraction for carmustine for strain data was twenty% on P-388, 50%–100% on 50-1210, and 75% on CC. Ormustine also shows a high therapeutic effect on the B-xvi melanoma and the soft Lewis epidermoid carcinoma LLC. The tumor growth inhibition (TGI) on the B-16 melanoma achieves 99.3%–91% inside fifteen days (the increment in lifespan is 84%) and 99.9%—87% inside 14 days on LLC (the increase in lifespan is 84%). On carmustine, TGI by the B-16 melanoma comes upwardly to 89% and a 25%–60% past LLC.
When comparison data on the specific activity of ormustine with other NU derivatives, information technology becomes obvious that this drug possesses a stronger antitumoral action on a series of tumors.
A cytotoxicity inquiry was conducted on the Mel Z cellular line of disseminate melanoma of a human subject. The results of the research are given in Table 5.
As the table shows, ormustine possesses a more expressed cytotoxic activeness in comparison with other drugs.
three. Materials and Methods
three.1. Preparations and Reagents
The following substances were obtained for this study: ormustine substation (I. Ya. Postovsky Institute of Organic Synthesis of the Ural Co-operative of the Russian Academy of Sciences, Russia), mannitol (Chimmed, Russia), Kollidon 12 PF, Kollidon 17 PF, Kollisolv PEG-400 (BASF The Chemic Company, Frg), dextran Grandr~seventy,000 (Sigma-Aldrich GmbH, Germany), PEG-1500 (Chimmed, Russia), 99% sorbic acid (Fluka, Frg), 99% glutamic acid (Merck, Germany), ascorbic acid, reagent class (Chimmed, Russia), citric acid anhydrous, reagent grade (Ctrobel, Russia), potassium phosphate monobasic, reagent grade (Chimmed, Russia), sodium phosphate dibasic 12-water, reagent grade (Chimmed, Russian federation), hydrochloric acid, reagent grade (CJSC Mosreaktiv, Russia), 5% glucose solution for infusion (Biosynthesis, Russia), Hemodesum-N solution for infusion (Biok, Russia), Ringer's solution for infusion (CJSC "Rester", Russian federation), and 0.ix % sodium chloride solution for infusion (B. Braun Melsungen AG, Germany).
3.ii. Equipment
The following equipment was used for this study: a Scales Sartorius LA 1200 S (Sartorius AG, Germany); analytical scales Ohaus Belittling Plus 119 (Ohaus, USA), an ultrasonic bath Transsonic (Elma, Frg), a mechanical overhead Stirrer RZR 2021 Heidolph with a propeller stirring chemical element PR 30 Heidolph (Heidolph, Germany), a glass filter holder Millipore (Millipore, France) with nylon PALL N66 membrane filters with a diameter of 47 mm and a pore size of 0.22 microns (Pall Corporation, U.s., LLC Pall Eurasia, Russia), a freeze drying apparatus Minifast Practise.2 (Edwards, Great britain), a spectrophotometer Cary 100 (Varian, Inc., Commonwealth of australia), a pH-meter HANNA pH 211 (Hanna Instruments, Germany), and a Dispensette dispenser (Make, Deutschland).
3.3. Study of the Solubility of Ormustine Substance
The study of the solubility of the ormustine substance was conducted visually at xx ± 2 °C using a multifariousness of solvents and expressed every bit a per centum by weight/volume [11].
3.4. Sterilizing Filtration of Ormustine Solution
Sterilizing filtration was carried out under vacuum using a nylon (filtration using glass filter holder Millipore) and polyethersulfone (included on Stericup vacuum filtration system) membrane filter with a pore diameter of 0.22 microns.
iii.5. Freeze Drying of Ormustine Solution
Freeze drying of DS was carried out on a freeze-drying apparatus Minifast DO.2. Thus, ormustine solution was filtered immediately after preparation and dosed with v mL ormustine solution (empirically established optimum filling volume) in vials of 20 mL, loaded onto the freeze drying shelves, and lyophilized using three different temperature conditions:
-
ormustine solution loading in the bottle on warm shelves with the rapid freezing and uniform temperature rise;
-
ormustine solution loading in the bottle on warm shelves with the ho-hum freezing and uniform temperature rise;
-
ormustine solution loading in the canteen on common cold shelves with the rapid freezing and uniform temperature rise.
three.6. Potentiometric Determination of pH of Solution and Lyophilizate (upon Rehydration) of Ormustine
The decision of pH of the solutions and the lyophilizate (upon rehydration) of ormustine was carried out potentiometrically.
3.7. Quantification of Ormustine in Solution and Lyophilizate
The content of DS in the solutions and lyophilizate was determined spectrophotometrically using a working standard sample substance of ormustine at λ = 396 ± 2 nm.
Dilution of the fresh ormustine solution was performed every bit follows:
The separation of insoluble ormustineis was carried out past filtering of the solution via sterile nylon membranous filters. Five milliliters of the filtered ormustine solution was transferred with a measuring pipette to a measuring flask with a capacity of l mL, brand up the book of the flask with 0.01 One thousand hydrochloric acidum, and mixed.
Lyophilizate dilution was performed every bit follows:
The contents of the flask were dissolved with 0.01 M hydrochloric acid and quantitatively transferred to a measuring flask with a capacity of fifty mL. The solution volume was brought to a tag by the same dissolvent and mixed. The time betwixt the get-go of the preparation of the test solution and the measurement of its optical density was not to exceed 30 min.
Preparation of the standard solution was performed every bit follows:
About 125 mg (precise exam portion) of ormustine substance was dissolved with 0.01 One thousand hydrochloric acid and quantitatively transferred to a measuring flask with a capacity of 50 mL. The solution book was brought to a tag by the same dissolvent and mixed. The fourth dimension between the beginning of the preparation of the test solution and the measurement of its optical density was non to exceed 30 min.
The optical density of the test solution and the standard solution was measured on the spectrophotometer in the assimilation maximum at a wavelength of 396 ± ii nm in the cuvette with a layer thickness of 10 mm, with 0.01 M hydrochloric acid used as a comparison solution.
The ormustine contents X (mg) in the flask were calculated with the post-obit formula:
X = (D1 × Vi × a0)/(D0× 50),
where Done = the optical density of the exam solution; D0 = the optical density of the standard solution; Vane = the size of dilution of the exam solution; V0 = the size of dilution of the standard solution; a0 = a precise test portion of a standard sample, in mg.
four. Conclusions
In the course of this research, aimed at developing a novel injectable NU ormustine PF, a solvent was selected, namely a 0.ane One thousand solution of hydrochloric acid, which was constitute to significantly increase the solubility of the substance, and the use of ultrasound in the technological process of obtaining the ormustine solution was demonstrated. In club to produce a PF stable in time, a lyophilization regime was designed. For constructive application of this procedure afterward a series of experiments, Kollidon 17 PF was chosen with a concentration of half-dozen% equally a shaper, which allowed obtainment of a high-quality lyophilizate. At the final stage, nosotros estimated the effects of solvents for the rehydrating of lyophilized ormustine DS on its stability; as a result, we institute that the solvents that ensure the most long-term stability of the resulting DS solution and that practice not cause astute toxicity are a five% glucose solution, a phosphate buffer solution, and a 0.9% NaCl solution. Equally a result of complex technological research, we adult stable lyophilized ormustine DS, which was then transferred to preclinical studies. The received results of the preliminary preclinical trials provide grounds for the continuation of the enquiry of ormustine, for the study of its cross-resistance with other NU derivatives, and, via combination therapy of tumors, for the rise in efficiency of the treatment of oncologic patients.
Acknowledgments
All studies were carried out in the framework of the State contract No. 13411.1008799.13.163 "Preclinical anticancer nitrosourea form of drug."
Author Contributions
Natalia Oborotova and Natalia Bunyatyan conceived and designed the experiments; Ludmila Nikolaeva, Ekaterina Sanarova, and Anna Lantsova performed the experiments; Xi Zhang, Olga Orlova, and Alevtina Polozkova analyzed the data; Ludmila Nikolaeva and Ekaterina Sanarova wrote the paper.
Conflicts of Interest
The founding sponsors had no office in the design of the written report; in the collection, analyses, or interpretation of information; in the writing of the manuscript, and in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| PF | pharmaceutical formulation |
| NU | nitrosourea |
| DS | drug substance |
| CC | cervical cancer |
| TGI | tumor growth inhibition |
References
- Oborotova, N.A.; Ryshkova, N.E.; Smirnova, Z.Due south.; Polozkova, A.P.; Orlova, O.L.; Shprakh, Z.Southward.; Peretolchina, N.M.; Khalanskii, A.South.; Bagirova, V.Fifty.; Yu, A. Biopharmaceutical investigation of a new medicinal course of the antitumor drug one,iii-Bis(two-chloroethyl)-one-nitrosourea. Pharm. Chem. J. 2001, 35, 108–111. [Google Scholar] [CrossRef]
- Lantsova, A.5.; Sanarova, E.V.; Oborotova, Northward.A. Antineoplastic preparations derivative of nitrosoalkylurea for handling of cancer neoplastic of dissimilar genesis. J. Biopharm. 2014, 6, 38–51. [Google Scholar]
- Kim, J.C.; Lim, Y.G.; Min, B.T.; Park, J.I. Grooming of N′-substituted anilino-N-methyl-Due north′nitrosoureas as candidate antitumor agents. Arch. Pharm. Res. 1994, 17, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Gate, 50.; Kenneth, D. Alkylating Agents. In Cancer Direction in Man: Chemotherapy, Biological Therapy, Hyperthermia and Supporting Measures; Springer: Berlin, Federal republic of germany, 2001; Book xiii, pp. 61–85. [Google Scholar]
- Prestayko, A.Westward.; Baker, L.H.; Crooke, S.T. Nitrosoureas: Current Status and New Developments; Academic Press: New York, NY, Usa, 2013; p. 436. [Google Scholar]
- Lantsova, A.; Kotova, Due east.; Sanarova, K.; Oborotova, N.; Poloskova, A.; Barushnikov, A.; Orlova, O.; Krasnov, V. Biopharmaceutical report of nanostructured conception of the anticancer drug derivative of nitrosoalkylurea lysomustine. J. Drug Deliv. Sci. Technol. 2012, 22, 469–472. [Google Scholar] [CrossRef]
- Baryshnikova, K.A.; Albassit, B.; Saprykina, N.S.; Levit, G.L.; Matveeva, T.5.; Krasnov, Five.P. Antitumor activeness of new compounds from the form of nitrosoalkylurea. Russ. J. Biother. 2013, 12, 8. [Google Scholar]
- Grischenko, N.V.; Albassit, B.; Baryshnikova, M.A.; Lantsova, A.Five.; Polozkova, A.P.; Oborotova, N.A.; Krasnov, V.P.; Baryshnikov, A.Y. Cytotoxic outcome of anticancer drug formulations belonging to nitrosourea subclass. Russ. J. Biother. 2014, 13, 49–53. [Google Scholar]
- Saprykina, North.S.; Baryshnikova, Yard.A.; Krasnov, V.P.; Levit, G.L.; Lantsova, A.Five.; Sanarova, Eastward.5.; Oborotova, N.A.; Baryshnikov, A.Y. Antitumor activeness of the chemical compound OR-2011 to mice transplanted melanoma. Russ. J. Biother. 2014, 13, 125. [Google Scholar]
- Smirnova, Z.S.; Boricova, 50.Thou.; Kiseleva, M.P.; Saprykina, N.Southward.; Krasnov, V.P.; Levit, G.L.; Matveeva, T.V.; Lantsova, A.V.; Sanarova, E.V.; Orlova, O.L.; Polozkova, A.P. The antitumor activeness of nitrosourea OR-2011 to mice lymphocytic leukemia. Russ. J. Biother. 2014, 13, 130. [Google Scholar]
- Sanarova, East.V.; Lantsova, A.V.; Nikolaeva, Fifty.L.; Polozkiva, A.P.; Orlova, O.L.; Oborotova, N.A.; Musiyak, Five.Five.; Levit, G.L.; Krasnov, 5.P. The report of the solubility of the new compound course nitrosoalkylurea to create injection formulation. Russ. J. Biother. 2014, 13, 125. [Google Scholar]
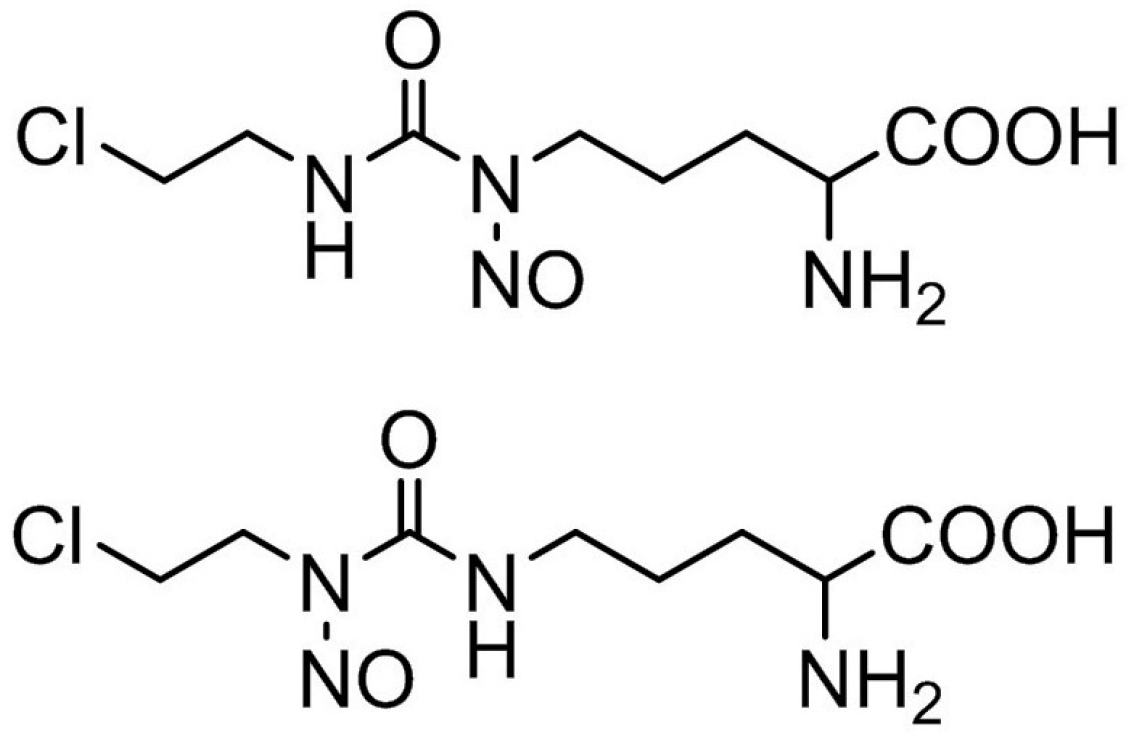
Effigy 1. Structural formula of ormustine.
Effigy 1. Structural formula of ormustine.
Effigy 2. Temperature curve for conception during drying.
Figure 2. Temperature curve for conception during drying.
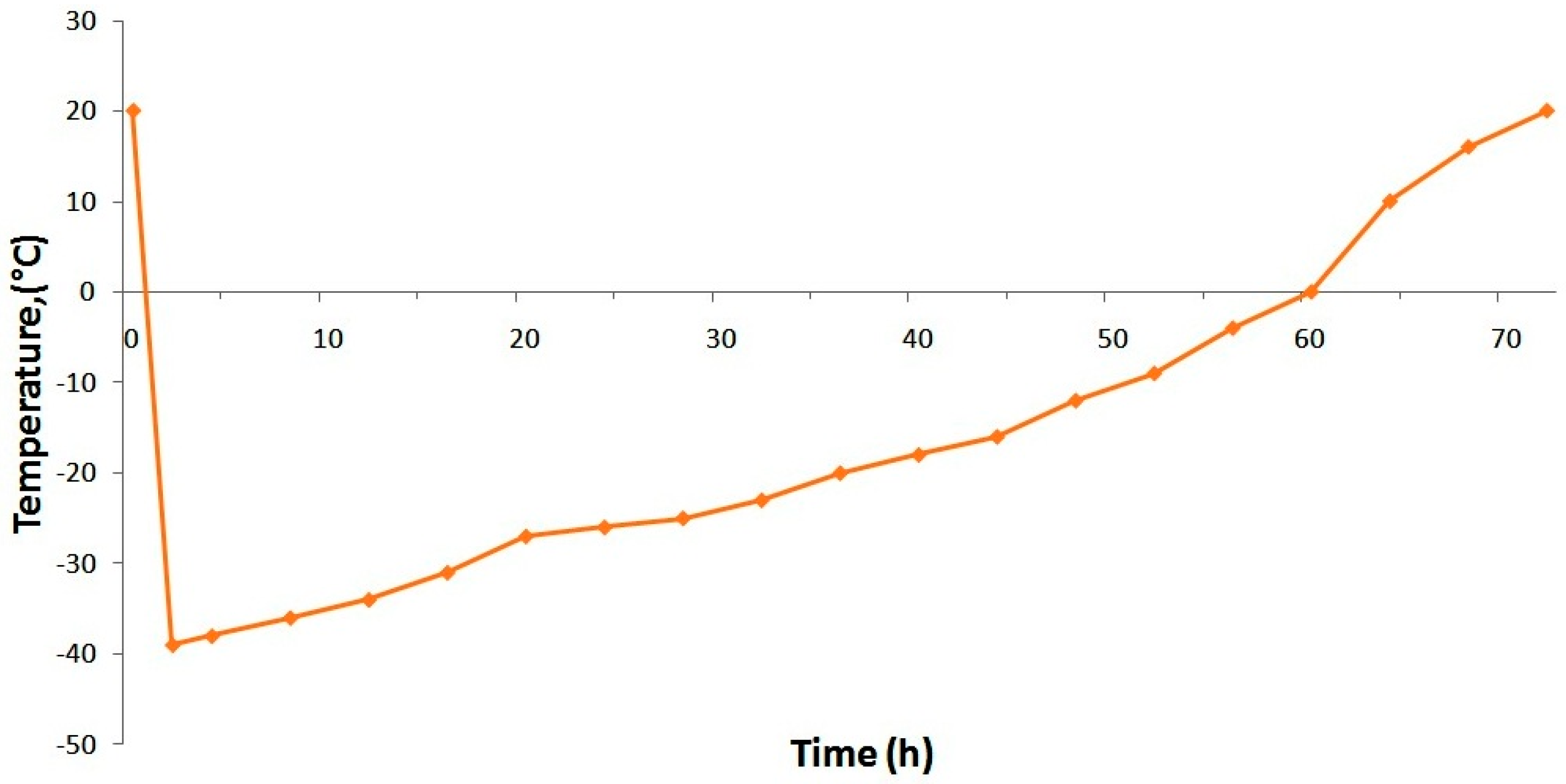
Figure iii. Stability studyof ormustine in PF after rehydration in various solvents.
Figure 3. Stability studyof ormustine in PF after rehydration in various solvents.
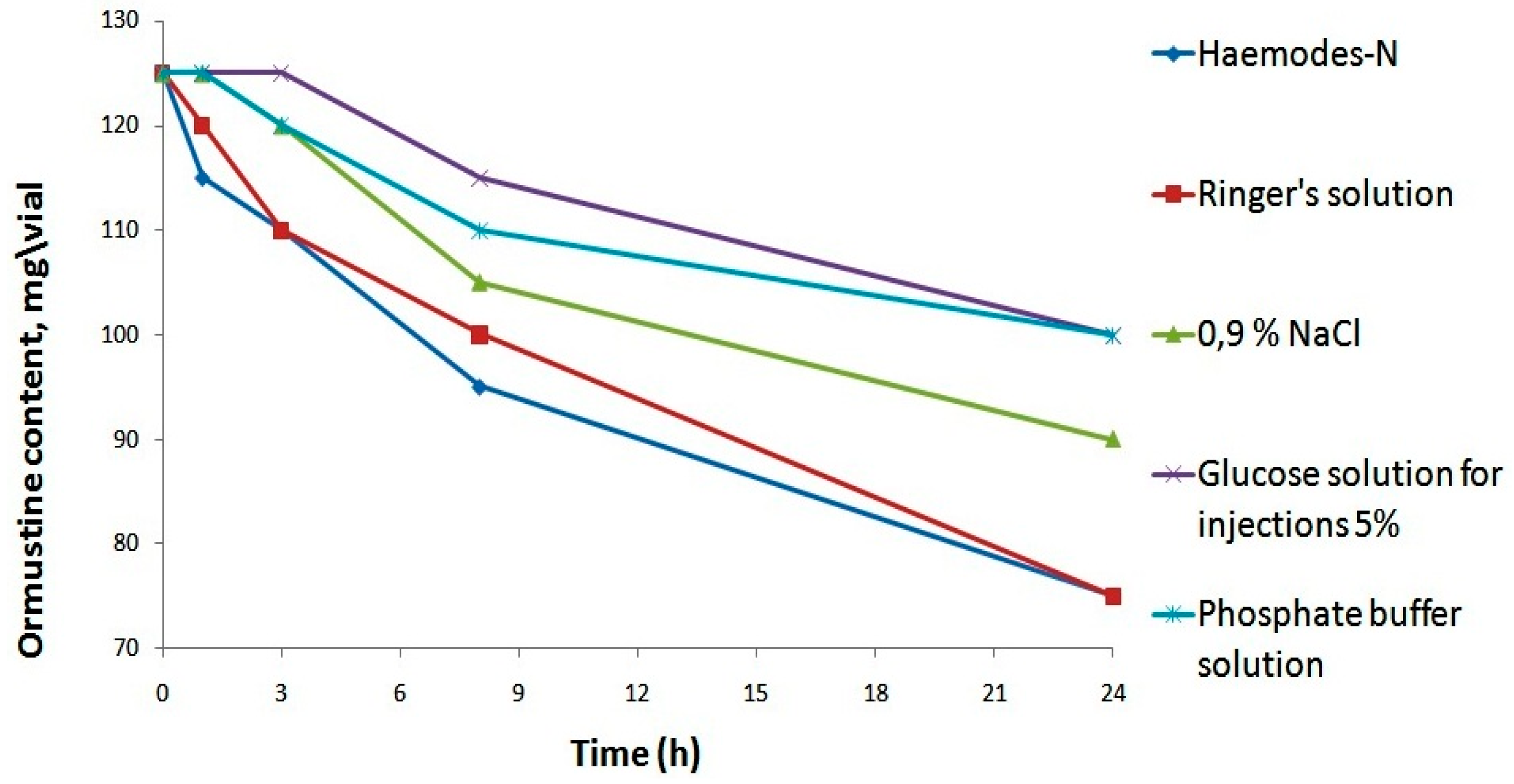
Table i. Solubility of ormustine substance in h2o and solutions of solubilizers.
| Solvent | pH | Ormustine Concentration in Solution, % |
|---|---|---|
| water for injection | three.9 | 1.0 |
| 5% solution of mannitol | 3.five | 1.seven |
| 2% solution of Kollidon 17 PF | 3.v | 1.eight |
| 2% solution of dextran | iii.6 | 1.7 |
| 5% solution of mannitol two% solution of Kollidon 17 PF | 3.6 | 1.vi |
| 0.two% solution of citric acid | 2.9 | 1.v |
| 2% solution of citric acrid | 2.4 | two.0 |
| 4% solution of citric acid | 2.3 | ii.five |
| 6% solution of citric acid | two.2 | 2.5 |
| x% solution of Kollidon 17 PF 0.one% solution of citric acid | 3.ane | 1.six |
| 10 % solution of Kollidon 12 PF 0.1% solution of citric acid | 3.two | i.7 |
| ten% solution of Kollisolv PEG-400 0.ane% solution of citric acid | three.1 | 1.8 |
| 10% solution of PEG-1500 0.1% solution of citric acid | iii.ii | i.8 |
| 0.1 M solution of hydrochloric acid | 2.0 | 2.5 |
Table two. Upshot of different methods on the rate of dissolution of the ormustine substance.
| Parameter | Dissolution Method | |||
|---|---|---|---|---|
| Heating | Ultrasound Treatment | Magnetic Stirrer | Propeller Stirrer | |
| median dissolution rate, g/min | 0.7 ± 0.1 | 0.89 ± 0.05 | 0.16 ± 0.05 | 0.35 ± 0.i |
| ormustine content in the solution after complete dissolution, % of the theoretical content | 75 ± 5 | 99 ± ii | 85 ± 3 | 89 ± three |
Tabular array 3. The touch on of shapers on the stability of ormustine.
| Shaper | Dissolution Time, min | pH | DS Loss inside 3 h, % |
|---|---|---|---|
| - | 12 | 2.0 | vii.iii |
| Kollidon 17 PF half-dozen% | 17 | two.ane | ane.four |
| Kollidon 12 PF 10% | 25 | 2.1 | eleven.5 |
| Kollidon 12 PF 6% citric acid 0.1% | 25 | ii.1 | 6.4 |
| Kollidon 12 PF 4% lactose 2% | 15 | 2.0 | 9.eight |
| lactose 4% | xvi | 2.0 | 19.half dozen |
| mannitol iv% | 22 | two.0 | 16.1 |
Table four. Changing of pH by adding diverse amounts of solvents to lyophilized ormustine PF.
| Volume of Added Solvent, mL | pH | ||||
|---|---|---|---|---|---|
| 0.9% NaCl | 5% Glucose Solution | Ringer's Solution | Hemodes-N | Phosphate Buffered Saline | |
| 5 | ii.0 | 2.0 | ii.0 | ii.iii | non dissolved |
| x | 2.0 | ii.1 | 2.1 | ii.5 | vi.5 |
| 15 | 2.1 | two.2 | ii.3 | 2.9 | - |
Table 5. Studying influence of nitrosoureas (NUs) on the Mel Z line cells.
| Drug | IC50 (mg/mL) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| Aranoza | 0.9 | 0.ix | 0.225 |
| Lizomustine | 0.125 | 0.125 | 0.062 |
| Ormustine | 0.125 | 0.062 | 0.062 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open admission commodity distributed under the terms and conditions of the Artistic Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Sterilizing Filters Have A Pore Size Of 0.22 Þâ¼m. Which Of The Following Statements Is True?,
Source: https://www.mdpi.com/1424-8247/9/4/68/html
Posted by: avishispers1979.blogspot.com


0 Response to "Sterilizing Filters Have A Pore Size Of 0.22 Þâ¼m. Which Of The Following Statements Is True?"
Post a Comment